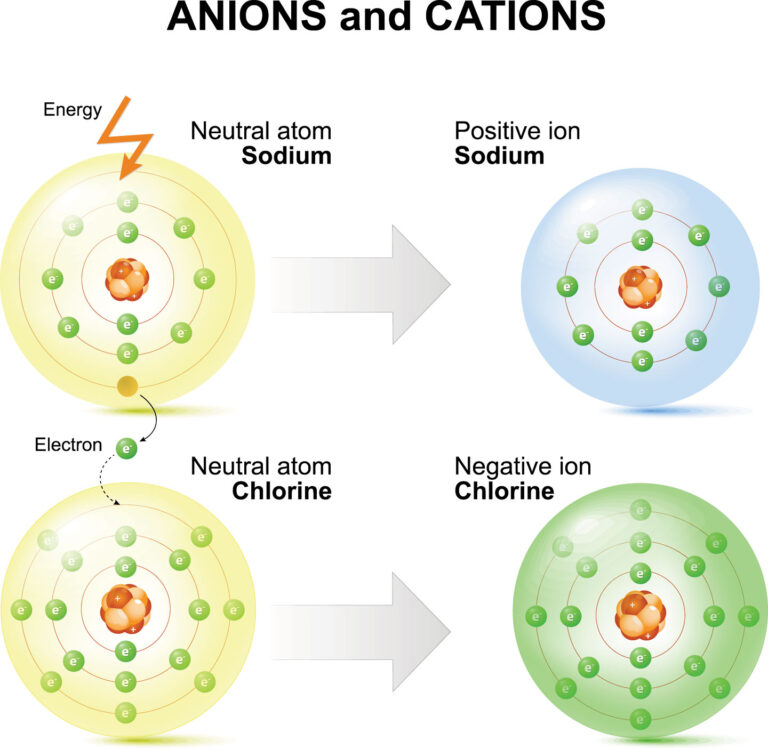
Atoms, the building blocks of all matter, undertake a fascinating transformation into ions under specific circumstances. This blog delves into the intriguing question: what event changes an atom into an ion? Understanding this phenomenon is crucial in various scientific and practical applications. Atoms become ions by gaining or losing electrons, resulting in a charged particle with unique properties. The process is pivotal in chemistry, physics, and even everyday occurrences. Join us on a journey to uncover the mechanisms behind this fundamental alteration and grasp the significance of ions in the world around us.
Introduction: Exploring the Concept of Atoms and Ions
Atoms are the fundamental building blocks of matter, consisting of protons, neutrons, and electrons. However, when atoms gain or lose electrons, they transform into ions, charged particles that play crucial roles in various chemical reactions and biological processes. In this section, we delve into the intriguing realm of atoms and ions to understand what event changes an atom into an ion.
The Structure of Atoms
Atoms contain a nucleus composed of protons and neutrons, surrounded by electrons orbiting in specific energy levels. These electrons determine the chemical properties of an element.
When atoms are in their neutral state, the number of protons equals the number of electrons, resulting in a balanced charge.
Formation of Ions
When atoms gain or lose electrons, they become ions with a net positive or negative charge. This process occurs during chemical reactions or interactions with other atoms, ions, or electromagnetic radiation.
- Cation Formation: Atoms that lose electrons become positively charged cations.
- Anion Formation: Atoms that gain electrons become negatively charged anions.
The Nature of Atoms: Understanding the Basic Building Blocks
Atoms are the fundamental units of matter, composed of protons, neutrons, and electrons. These tiny particles are the building blocks of all elements and play a crucial role in the formation of compounds and molecules.
The Structure of an Atom
An atom consists of a dense nucleus containing positively charged protons and neutrally charged neutrons, surrounded by negatively charged electrons orbiting in energy levels or shells.
The protons and neutrons are located in the nucleus, while electrons move around the nucleus in specific paths.
Atomic Number and Mass Number
The atomic number of an atom represents the number of protons in its nucleus, defining the element.
The mass number is the sum of protons and neutrons present in the nucleus, determining the atomic mass of the atom.

Ion Formation: Explaining the Transformation Process
When it comes to understanding what event changes an atom into an ion, it is essential to delve into the process of ion formation. An ion is formed when an atom gains or loses one or more electrons, resulting in a net positive or negative charge.
The Role of Electrons
Atoms become ions through the process of ionization, which involves the interaction of electrons with atoms. This process can occur through various mechanisms such as chemical reactions, radiation, or electric discharge.
Types of Ions
There are two main types of ions: cations and anions. Cations are positively charged ions that result from atoms losing electrons, while anions are negatively charged ions that form when atoms gain electrons.
Factors Affecting Ionization: Delving Into the Variables
When considering **what event changes an atom into an ion**, various factors play crucial roles in the ionization process. Among the key variables affecting ionization are:
Atomic Structure
The arrangement of electrons in the atom’s energy levels significantly impacts its ionization potential. Atoms with loosely bound electrons are more prone to losing them and forming ions.
The image below illustrates the electron cloud model of an atom, showing the distribution of electrons around the nucleus.
Nature of Electrons
Electrons with higher energy levels are easier to remove from an atom, leading to lower ionization energy. This characteristic is fundamental in understanding which elements are more likely to form ions under specific conditions.
The Role of Electrons: Unveiling the Key Player
Electrons play a crucial role in determining the chemical behavior of atoms, especially when it comes to the transformation of an atom into an ion. Understanding how electrons function is key to unraveling the mystery of this process.
The Movement of Electrons
Electrons move around the nucleus of an atom in specific energy levels or shells. When an atom undergoes a change to become an ion, it involves the gain or loss of electrons.
Ion Formation
When an atom gains or loses electrons, it becomes an ion with a positive or negative charge. This transformation occurs due to interactions with other atoms or energy sources.
- Positive Ion (Cation): Forms when an atom loses electron(s).
- Negative Ion (Anion): Forms when an atom gains electron(s).
Common Ionizing Events: Examining the Catalysts
Ionization, the transformation of an atom into an ion, can occur through various processes triggered by specific events. Understanding these catalytic events is crucial in unraveling the mystery of atomic behavior.
Impact of Radiation
Radiation exposure is a significant catalyst for ionization. When high-energy radiation interacts with an atom, it can dislodge electrons, resulting in the formation of ions. This process is commonly observed in nuclear reactions.
Chemical Reactions
Chemical reactions also play a vital role in ionizing atoms. During these reactions, atoms can gain or lose electrons, leading to the creation of ions. This phenomenon is fundamental in various industrial processes and natural phenomena.
Applications of Ionization: Real-world Implications
Ionization, the process of converting neutral atoms into ions by adding or removing electrons, has numerous real-world applications across various industries. Let’s explore some of the key implications of ionization in today’s world.
1. Ionization in Healthcare
In the medical field, ionization plays a crucial role in radiation therapy for cancer treatment. Ionizing radiation is used to target and destroy cancer cells effectively.
2. Ionization in Environmental Science
Ionization detectors are used to measure air quality and detect pollutants in the atmosphere. These devices help in monitoring environmental conditions and ensuring public health safety.
3. Ionization in Consumer Electronics
Modern electronic devices like smartphones and laptops utilize ionization processes to create conductive paths that enable the flow of electricity.
Frequently Asked Questions
-
- What is an atom?
- An atom is the basic unit of matter, consisting of protons, neutrons, and electrons.
-
- What is an ion?
- An ion is an atom or molecule that has gained or lost electrons, giving it a positive or negative charge.
-
- What event changes an atom into an ion?
- An atom changes into an ion when it gains or loses electrons through a process called ionization.
-
- How does ionization occur?
- Ionization can occur through various processes such as radiation, chemical reactions, or electrical discharges.
-
- What are the properties of an ion?
- Ions have different properties than their parent atoms, such as being able to conduct electricity and participate in chemical reactions.
Unraveling the Enigma: The Transformation from Atom to Ion
Through our exploration into the intriguing world of atoms and ions, we have discovered the pivotal event that changes an atom into an ion – the gaining or losing of electrons. This fundamental process alters the charge and reactivity of the atom, leading to a shift in behavior and properties. Understanding this transformation provides profound insights into the behavior of ions in chemical reactions and everyday life. As we conclude our journey, remember that the conversion of an atom into an ion is not just a scientific phenomenon but a captivating revelation of the dynamic nature of matter. Embrace the wonder of this transformation and continue to delve deeper into the mysteries of the atomic world.